Who are we?
We are a technical Full Service CRO that offers end-to-end Clinical Trial Services, complemented by an industry-leading suite of specialized Pharmacovigilance Solutions. Our flexible working models are designed to seamlessly adapt to client requirements, whether you require functional solutions or global studies or tailored on-site and off-site support.

Different models to meet diverse needs
What makes our Solutions Industry Leading?
With multiple facilities in India and the USA, we serve a truly global footprint. At our organization, we are committed to extending our capabilities to provide optimal solutions that leverage local expertise to suit global programs, while accelerating every stage of the drug development process.

Oncology Focused CRO
Deep expertise in planning and executing Oncology trials across a wide range of complex indications and sites

Risk Management & Medical Safety concentrated experts
Systems & Capabilities in managing products with complexities and high end PV Needs Globally: such as Risk Measures, REMS, Complex PBRER
NCEs, Biosimilars & Vaccines
Global Multicentric clinical trials for NCEs, Biosimilars, and Vaccine molecules to accelerate market entry

Regulatory Solutions Across Global Markets
Robust submission strategies and compliance support for USFDA, EMA, WHO, and DCGI
Catalyzing Success: Prioritizing Timeliness, Patient Diversity & Site Network
At COD, we believe that timeliness, patient diversity, and a robust site network are the key catalysts for success in clinical trials. Our commitment to these three pillars drives us to deliver high-quality results efficiently, ultimately accelerating the development of life-changing therapies.
130+
Clinical Trials experience across Globe40+
Active Clients Globally1500+
Investigators DatabasePartnering for Safety: Delivering Quality Global Pharmacovigilance Solutions
We deliver quality-driven global pharmacovigilance services that prioritize patient safety and regulatory compliance. By emphasizing collaboration and flexibility, we address our customers’ unique needs. Our robust Risk Management and comprehensive Medical Information services ensure safety issues are identified and addressed, supporting informed decision-making for all stakeholders.
1800+
Medical Safety Reports Generated
1023 +
Products Safety Activities (Drug, Device & Combination products)
112+
Countries Safety Support Capabilities
102350+
ICSRs Managed with 8+ Data Migration
Featured Solutions
Clinical Development
Clinical development is a crucial phase in bringing new therapies to market, and at COD, we offer a comprehensive approach that integrates regulatory strategy and planning, protocol development, medical writing, key opinion leader engagement, and statistical analysis plans.
Read MoreCombining Experience & Expertise across Therapeutic Areas for Enhanced Clinical Outcomes
Learn More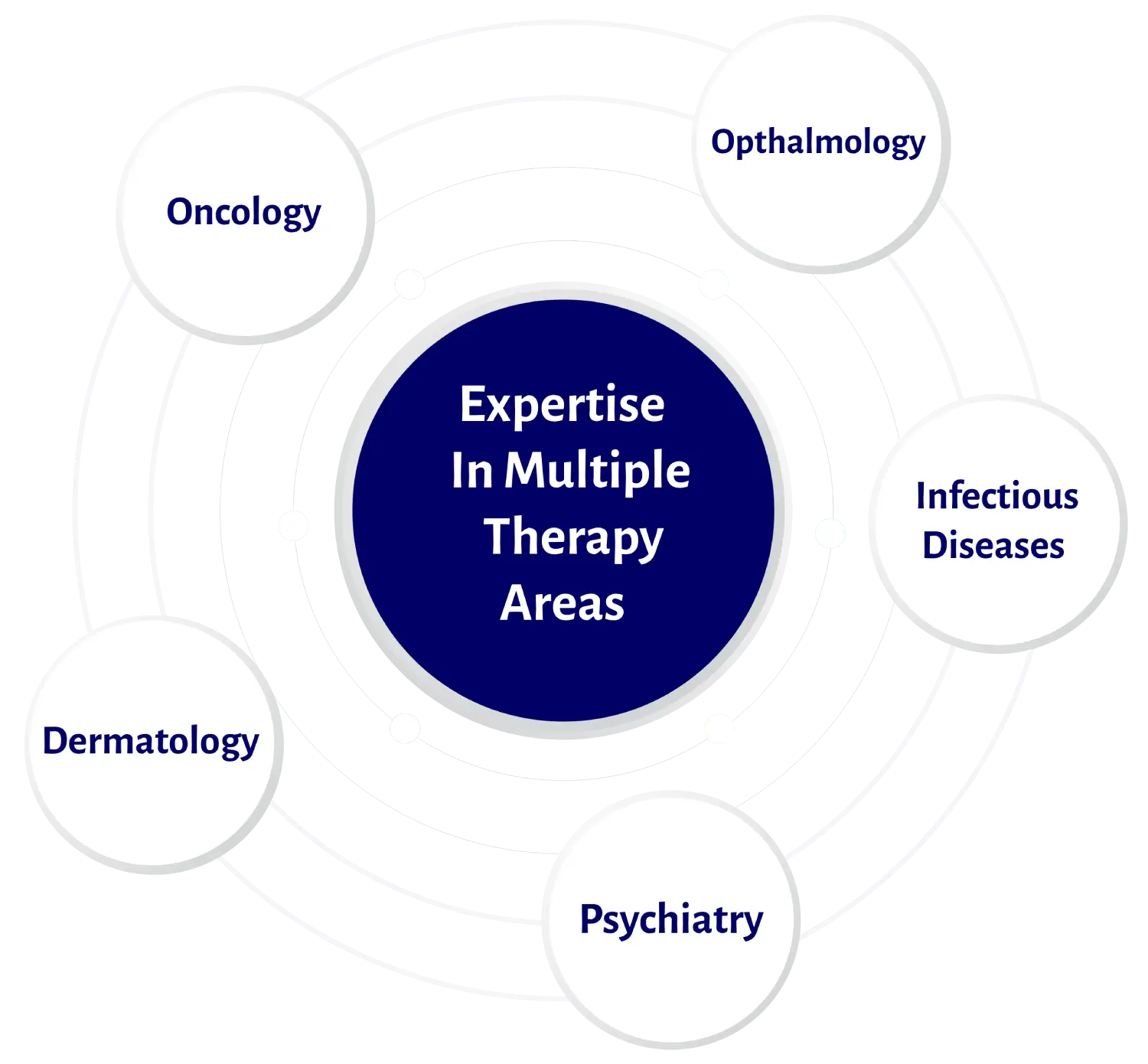
Request for Proposal
We provide a comprehensive full-service suite of specialized CRO services. Trust the experts at COD Research to efficiently navigate regulatory approvals and expedite the market entry of your drug or device. Connect with us today to discuss how we can support your clinical development needs.