The article is useful for:
- PRH/MAH In Malaysia (MY)
- Global PV Service Provider (PVSP) for MY PRHs Local PVSP in Malaysia
- Regulatory Affairs Consultants/Service Providers Malaysian RPPV
Background:
NPRA has released an updated PV guidance (GVP Aug-2021) on 06- Sep-2021, with specific inclusion of one new part (Part 6) related to Pharmacovigilance System Master File (PSMF) and Pharmacovigilance System Summary (PVSS).
This guideline is adopted from the EU Good Pharmacovigilance Practices (GVP) Module II and other PV-related guidelines by other regulatory agencies. The guidance provides details regarding the requirements for PSMF in Malaysia including its maintenance, content and associated submissions to the Authority.
1. When to submit PVSS and PSMF?
When an assessment of the pharmacovigilance system is warranted, such as prior to Good Pharmacovigilance Practice Inspection (GVPI).
As per the requirement of Conditional Registration of Pharmaceutical Products during Disaster
The timeline of submission will be notified by the NPRA
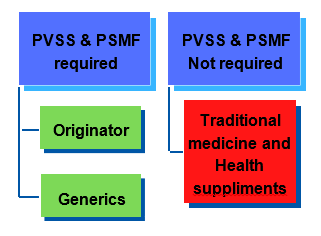
2. Any product exemption from PVSS and PSMF?
All PRHs (Originator or Generics) are required to prepare both PVSS and PSMF.
Until further announcement, PVSS and PSMF for Traditional Medicines and Health Supplement (TMHS) products are not required to be prepared and submitted. However, it is expected that PRH will continue to maintain and monitor the pharmacovigilance activities for TMHS products.
3. Pharmacovigilance System Summary (PVSS)
PVSS similar to 1.8.1 (Summary of PV System) mentioned in EU GVP Module, with some localflavor.
It briefly describes or summarises the pharmacovigilance system of thePRH in Malaysia. The PVSS serves as a commitment document by the PRH to develop a PSMF and to establish a PV system within the company.
Why PVSS is required?
The PVSS is a part of the requirements to be fulfilled by a PRH as laid out in the Guidance and Requirements on Conditional Registration of Pharmaceutical Products during Disaster. Apart from this, the PVSS may also be used by the Authority for risk-based assessment to prioritize PRHs for inspection in GVPI.
What is the frequency and format of PVSS submission?
PVSS is expected to be submitted only once for each PRH, unless requested by the Authority. No maintenance of PVSS is expected from the PRH. The submission of PVSS should be in hard copy.
Is there any template available for PVSS?
The NPRA has provided a sample of PVSS under Appendix 7 and can be referred from below mentioned link:
4. Pharmacovigilance System Master File (PSMF)
The PSMF is a detailed description of the pharmacovigilance system used by PRH to fulfil its pharmacovigilance tasks and responsibilities designed to monitor registered products’ safety and any changes to their benefit-risk balance.
Why PSMF is required?
The PSMF is applicable for any human medicinal product registered in Malaysia, irrespective of the product registration procedure or marketing status (except for cosmetic products and veterinary products). Since PSMF provides detailed description of the pharmacovigilance system within a PRH, it reflects the PRH’s readiness and competency in pharmacovigilance. The PSMF could also provide general insights on the pharmacovigilance system of the PRH to the Authority.
How many PSMFs are required?
- One PSMF describes PV system for one or more medicinal products of one MAH
- If the PRH wishes to apply the same pharmacovigilance system for all categories of products, only one PSMF is required
- MAH may apply separate PV systems for different categories of products (vaccines, health supplements, etc.)
- In case of the different PV systems, describe each system in a separate PSMF
- One PV system may be shared by several MAHs Each MAH responsible for a PSMF has to describe the PV system applicable for its products
- MAH may delegate PV activity to a third party
- The PSMF of a MAH may cross-refer to all or part of the PSMF managed by the third party
- The MAH retains ultimate responsibility for the PV system
Any template of PSMF available?
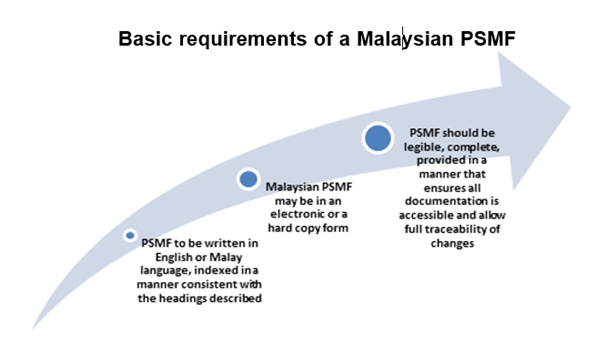
The content of the PSMF should be indexed following the modular system described in section P6.3 and the annex headings described in section P6.3.8. Detailedinformation that may change frequently could be referred to and contained in the Annexes. This can be referred from the below-mentioned link.
How to submit a PSMF?
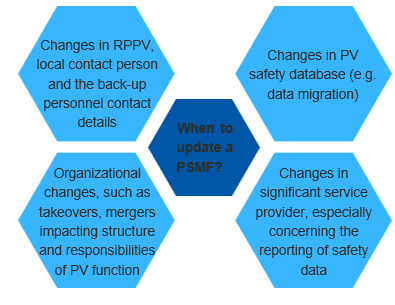
The PSMF content will continue to be updated on a periodic basis; however, any changes in the mentioned situations require a notification submission to the NPRA
5. Responsible Person for Pharmacovigilance (RPPV)
PRH must have permanently and continuously at its disposal an appropriately responsible person for PV (RPPV) activities, including developing and updating the PSMF.
RPPV should preferably be based in Malaysia.
When RPPV is based outside Malaysia (e.g., Singapore), a contact person is to be based in Malaysia. The local contact person must be contactable by Authority at all times, and the details of both the local contact person and the RPPV must be informed to Authority
PRHs are required to inform the Authority of any changes to the contact details of the RPPV, local contact person (if applicable), and all back-up personnel. The acceptable timeline to inform the Authority is within 30 calendar days from the effective date of the changes
During a GVPI, the RPPV and local contact person (if applicable) are required to attend the inspection
How COD can help?
Our SOP-driven processes ensure utmost compliance with the global/local requirements. For the PSMF update/annexure update perspective, we do cater risk proportionate method to have inbuilt procedural agility. Our experts understand the complexity of the PSMF and follow the regulatory guidelines on Good Pharmacovigilance Practice (GVP) specific to each PV system. This means PSMF is updated on a longer frequency for our clients having green KPI than red compliance clients. This is proven efficient and cost-effective for many of our clients and it also helps the QPPV to tab on red compliant PV system.
COD Research's PSMF capabilities
- Experts having >10 years of experience in PSMF Management
- Well versed with different regulatory requirements
- Worked on various territory specific templates for PSMF generation
- Able team members having >210 PSMF support exposure for Global clients
References:
-
Malaysian – GVP guidance:
-
Adverse Drug Reaction (ADR) / Adverse Event Following Immunization (AEFI) Reporting Manual for Healthcare Providers:
