Veterinary Pharmacovigilance in EU
An insight into databases, scope and access pre-requisitesAs committed in our last blog: Introduction and Concept of Veterinary Pharmacovigilance, we are sharing our second blog on insight into veterinary PV database/EU Portals to be utilized and getting access to these databases. This blog may contain already available information in regulation/guidelines, for which references are given at the end. The aim of the blog is quick learning and understanding the Veterinary PV from EU perspective.
Learning Objectives
- What is the different database/EU portals used in veterinary domain?
- What are the procedure and pre-requisite for getting different database access?
- What are the Implementation timelines for the different databases in EU?
Different databases involved in Veterinary Vigilance in EU
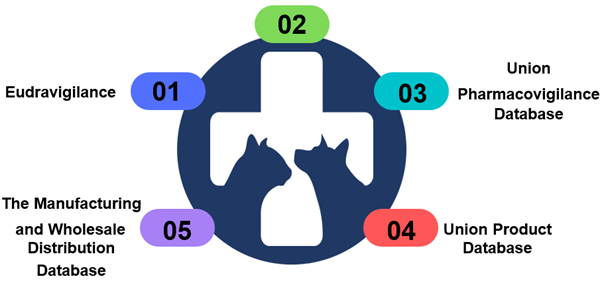
Let’s learn in detail about each of these database(s) with respect to Veterinary vigilance
1. Eudravigilance:
EudraVigilance is the data-processing network and database for managing and analysing information on suspected adverse reactions to medicines which have been authorised in the European Economic Area. Since Eudravigilance iscentralized system from where any MAH can access different other database hence this becomes the first pre-requisite for MAH to have account in EMA.
Let’s look into pre-requisites for Eudravigilance access:
A. Identify a person from organization who should be having account in EMA. Once it is identified below details must be submitted to EMA in order to get account created
B. User's first and last name, mail id and phone no, company name, postal code, Company cover letter.
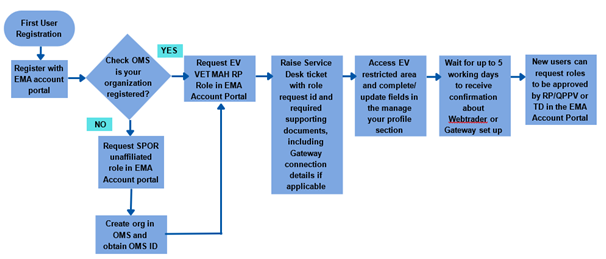
Once Account has been created, user can request for veterinary specific access. To get an access to Eudravgilance Vet below has to be ready with MAH.
- The organization is registered in the Organization Management System (OMS) as part of maintaining pharmacovigilance documentation
- Request role of EV VET MAH Responsible or EV VET NCA Responsible in the EMA Account Management portal by following steps mentioned above
Below is the overview of registration process for Eudravigilance veterinary for easy read
2. SPOR:
The SPOR are databases and services to manage the lists of controlled terminologies, which will be supplied in the electronic submission of veterinary medicinal product information into the UPD. What are the veterinary specific data available in SPOR? All Veterinary MAH need to have this account in order to add their organization details (OMS) and referential details (RMS). To get an access to SPOR, user must have active EMA account. By default, user will have SPOR unaffiliated user role. This role will enable the user to create their organization in this database.
3. Union Pharmacovigilance Database:
The Union Pharmacovigilance Database stores and makes available information on suspected adverse events for all veterinary medicinal products authorized in the Union.
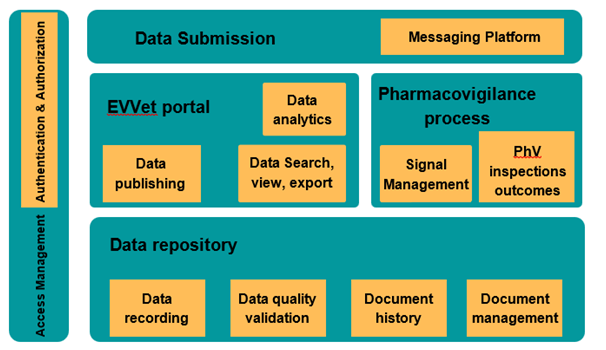
Reference: 131487_f215e50d-4231-48f2-b00d-a3e475b4a7b0 (5).pdf
4. Union product database:
The Union Product Database serves as a single source of information on all authorized veterinary medicines and their availability in EU and EEA Member States. Scope: Regulation (EU) 2019/6 mandates competent authorities (national competent authorities and the European Medicines Agency on behalf of the European Commission) to electronically submit and maintain information on all medicinal products for veterinary use into the UPD. The Regulation is applicable from January 2022 after a 3-year implementation period. What UPD contains or type of data stored: For marketing authorization holders, the Union Product Database provides self- service access for specific regulatory activities, including the management of variations that do not require assessment. It will also enable and simplify several regulatory procedures.
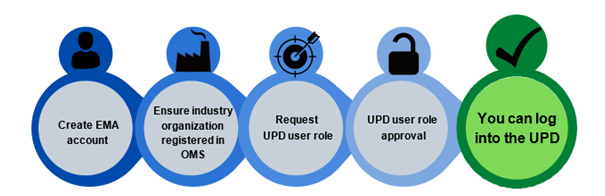
To get an access to UPD below pre-requisites needs to be taken care of:
- You must have an active EMA account
- The organization you represent must be recorded in EMA’sOrganisation Management Service (OMS)
- You have the appropriate UPD user role and affiliation to an organisation
5. Manufacturers and Wholesale Distributors database:
The requirements of MWD are implemented under EudraGMDP. EudraGMDP: EudraGMDP is an application portal that allows Inspectors of the European Economic Area (EEA) National Competent Authorities (NCA) to create and enter Good Manufacturing Distribution Practice (GMDP) Certificates (and Planned Inspections), GMDP Non-Compliance Reports, and Manufacturing/Importation Authorisations (MIA’s) into a Community database. In addition to the above functionality, the following modules are available for the data transfer from national systems: Wholesale Distribution Authorisations (WDA) Good Distribution Certificates (GDP) Statements of non-compliance with GDP Registration of manufacturers, importers and distributors of active substances for human use located in the EFTA
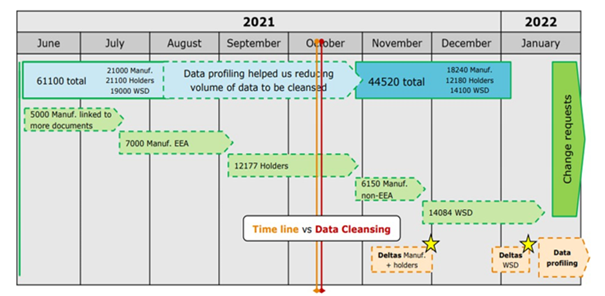
As of 28 January 2022, before applying for a new/updated manufacturing or wholesale distribution authorisation with national competent authorities, MAHs have to ensure that the organisation is correctly registered in OMS. Below infographic published by EMA have shown the time journey of integration of EudraGMDP and OMS.
Pre-Requisite:
- Eudra SSO (Single Sign-On) username and password
- Active EMA account to raise request for user Eudra SSO username and password
- Correct Organisation details added in OMS.
Abbreviation:
- SPOR: Substance Product Organization Referential
- EEA: European Economic Area
- UPD: Union Data product database
- EVVet3: Union Pharmacovigilance Database
- OMS: Organization Management Service
- NCA: National competent authorities
- MWD: The Manufacturers and Wholesale Distributors database
- EudraGMDP: Eudra Good Manufacturing and Distribution Practice
